New Drug Approvals
| 1 | Marzulene S Combination Granules | Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent (1968:Japan, 1990:China, 2000:India) “Granule mass-production technology” | |||
| Marzulene Combination Tablets 1.0ES | (Dosage form addition, 2003:Japan) | ||||
| Marzulene Combination Tablets 0.5ES | (Dosage form addition, 2008:Japan) | ||||
| Marzulene Combination Tablets 0.375ES | (Dosage form addition, 2009:Japan) | ||||
| 2 | AZULOXAⓇ Granules 2.5% | Gastric ulcer therapeutic agent (2001:Japan, 2004:Korea) | |||
| AZULOXAⓇ Tablets 15mg | (Dosage form addition, 2011:Japan) | ||||
| 3 | SuglatⓇ Tablets 25mg/50mg (Ipragliflozin (ASP1941) ) | Diabetes therapeutic agent/Selective SGLT2 Inhibitor (Type 2 Diabetes, 2014:Japan, 2015:Korea, 2019:Russia, Joint development with Astellas Pharma Inc.) (Type 1 Diabetes, 2018:Japan, Joint development with Astellas Pharma Inc.) | |||
| 4 | SUJANUⓇ Combination Tablets (Ipragliflozin/Sitagliptin (MK-0431J) ) | Type 2 Diabetes therapeutic agent/Selective DPP-4 inhibitor/Selective SGLT2 Inhibitor (2018:Japan, Joint development with Astellas Pharma Inc and MSD.) | |||
| 5 | XOSPATAⓇ Tablets 40mg (giliteritinib (ASP2215) ) | Antineoplastic drug/FLT3 Mutation |
 R&D Pipeline(2025.12)
R&D Pipeline(2025.12)
| 1 | ASP1570 | ①Development stage:Global/Phase Ⅰ ②Target:Cancer ③Development:Astellas | ||
| 2 | XOSPATA®(gilteritinib, ASP2215) | ①Development stage:Global/Phase Ⅲ ②Target:Acute myeloid leukemia ③Development:Astellas | ||
| ①Development stage:Global/Phase Ⅰ ②Target:ALK-positive non-small cell lung cancer (NSCLC) ③Development:Astellas | ||||
| 3 | KT6-971 | ①Development stage:Japan/Phase Ⅱ, Europe/Phase I ②Target:Dyslipidemia ③Development:Kotobuki | ||
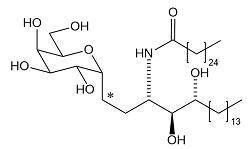
| 4 | KT7-533: α-C-Galactosyl Ceramide
| (Phase I in preparation:licensing-out) |
Medicinal Chemistry
Azulene Chemistry; Carbohydrate Chemistry; Transporter Inhibitor; Kinase Inhibitor;
Enzyme Inhibitor; Receptor Antagonist; Protein-Protein Interaction Inhibitor; Immunity
Pharmaceutical Engineering
Combination Granule; Combination Tablet; Easy-to-Swallow Tablet; Easy-to-Split Tablet (by fingers); Improved-Visibility Tablet;
Enteric-Coated Tablet; Patient-Friendly PTP Packaging (with improved visibility); Bitterness Reduction Technology; Miniaturized Tablet;
Capsule to Tablet Conversion; Kawara OD Tablet®; Film-Coated Orally Disintegrating Tablet (Coat OD Tablet®);
Gel-Coated Tablet (Gel-Coat Tablet®)
*Kotobuki Pharma retains all IP rights of “Kawara OD Tablet®” , “Coat OD Tablet®” and “Gel-Coat Tablet®”.