Starting with “Apricots”
 |  |
 |
Timeline list
・Misao Tomiyama, a representative employee and partner of Kotobuki Shokai, a limited partnership established in 1931, receives a license from Nagano Prefecture to manufacture and sell pharmaceuticals at the company’s Toyono Plant in Nagano City and establishes Kotobuki Seiyakusho.
The limited partnership, Kotobuki Shokai, had produced apricot jam, and produced apricot kernel water from apricot seeds, which were mostly waste1).
By cultivating herbal medicines in Nagano Prefecture, the company will also contribute to the preservation of the natural environment and the creation of job opportunities in Nagano Prefecture2).
Both 1) and 2) are based on the SDGs.

Apricot kernel water
・The company joins the Nagano Pharmaceutical Association (located in the Nagano Pharmaceutical Hall) under the trade name Kotobuki Seiyaku Co.and begins business activities.
・Kotobuki Pharmaceutical Co., Ltd. is registered as a corporation (capital of 300,000 yen).
・Misao Tomiyama took office as president.
・Joined Nagano Crude Drug Promotion Committee.
・Became the initiator of the establishment of the “Sakaki Medicinal plants Association”.

Company nameplate at the time of establishment
Lotus extract: EXTRACTUM SCOPOLIAE “KOTOBUKI”
・Moved the head office to 6351, Sakaki, Sakaki-machi.
・【Transformation of business from crude drugs to synthetic chemical pharmaceuticals.】A new “API plant with reinforced slate roof” will be built adjacent to the head office and the crude drug formulation plant for the production of chemically synthesized drugs.
Exterior view of API plant
・Opened Osaka Office.
・Begins production and sales of “Buformin Hydrochloride” for the first time as a chemically synthesized drug.
・Begins production and marketing of ”Clofibrate”, the first domestically produced ”Clofibrate”.
Clofibrate production facilities
・Begins production and marketing of protoporphyrin disodium and exports to Taiwan, South Korea and other overseas markets.
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE-S GRANULES in Japan.
(Activate drug discovery research on azulenes of Japanese origin)
Advertisement at the time of release
・QVF reactor introduced.
Completion of the first stage construction of Research Laboratory.



Panoramic view of the head office
・Completion of the second stage construction of Research Laboratory.
・Introduced high-speed automatic continuous packaging machine and continuous granulation dryer.
・Completion of research facilities of Radio Isotope.
・Our research paper published for the first time in Chemical & Pharmaceutical Bulletin.
・Founding 40th anniversary commemorative magazine “Housu” published.

・Our research paper published for the first time in Journal of Medicinal Chemistry.

・Additional set of drug substance manufacturing equipment in the south area of the second drug substance factory.
・Completion of the second pharmacy plant based on GMP.
・Research paper published for the first time in Bioorganic & Medicinal Chemistry Letters, a journal of the Elsevier’s.
・Kotobuki Pharmaceutical was selected as one of the referees for submitted papers in Elsevier’s “Bioorganic & Medicinal Chemistry Letters.”
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE-S GRANULES in India.
・Launched new Gastric ulcer therapeutic agent AZULOXA GRANULES (Egualen Sodium) in Japan.
・Introduced external lubricant spray device.
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE ES TABLETS (Dosage form addition) in Japan.
・Launched Gastritis, Gastric ulcer, duodenal ulcer therapeutic agent MARZULENE COMBINATION TABLETS 0.375ES (Dosage form addition) in Japan.
・Co-marketing of AZULOXA GRANULES 2.5%, a gastric ulcer treatment, with Ajinomoto Co., Inc.



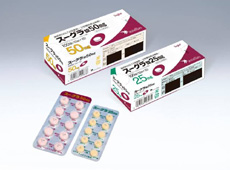
SGLT2 Inhibitor for Treatment of Type 2 Diabetes(Joint development with Astellas Pharma Inc.), in Japan.
Selective SGLT2 Inhibitor “Suglat Tablets 25mg, 50mg (Suglat, Ipragliflozin L-Proline)”
Drug combining selective DPP-4 inhibitor and selective SGLT2 inhibitor “SUJANU Combination Tablets (SUJANU, Sitagliptin phosphate hydrate, Ipragliflozin L-Proline)”
・Approved for manufacturing and sales in Japan, and the drug price is listed and released.
FLT3 Inhibitor “XOSPATA 40mg Tablets (XOSPATA, Gilteritinib)”
・Relocation of Tokyo Office.
・Research on Kawara OD Tablets®, which uses our unique formulation technology, was selected for the 2021 Nagano Prefecture Pharmacists Association Research Grant 21.
・FY2021 “Nagano Prefecture Pharmacists Association Research Grant 21: Voices of Joy from Award Winners (Representative Director and President Yasushi Tomiyama)” was published in the Nagano Prefecture Pharmaceutical Journal “Rindo”.
・Released Tramadol Hydrochloride OD Tablets 25mg/50mg “KO” in Japan.
This product is our “Kawara OD Tablets®”.

・The presentation ceremony for the Minister of Health, Labour and Welfare’s Letters of Appreciation to organizations cooperating in promoting blood donation activities for fiscal year 2023 was held.
・Tramadol Hydrochloride OD Tablets 25mg/50mg “KO” for the Treatment of Cancer and Chronic Pain
Recipient of the Encouragement Award for Invention at Kanto Region Local Commendation for Invention, organized by the Japan Institute of Invention and Innovation (JIII)![]()
Title of Innovation: Miniaturized Oral Tablet Designed for Water-Free Administration
・We began marketing its own single agent for “MARZULENE S GRANULES”, “MARZULEN COMBINATION TABLETS 1.0ES”, “0.5ES”, “0.375ES”, “AZULOXA GRANULES 2.5%” and “AZULOXA TABLETS 15mg” for gastritis and peptic ulcer treatment.